Grain boundaries are interfaces where crystals of different orientations meet within a material, significantly influencing its mechanical, electrical, and chemical properties. These boundaries can strengthen materials through grain boundary strengthening or lead to weaknesses such as corrosion and cracking. Discover how understanding grain boundaries can improve Your material performance by reading the full article.
Table of Comparison
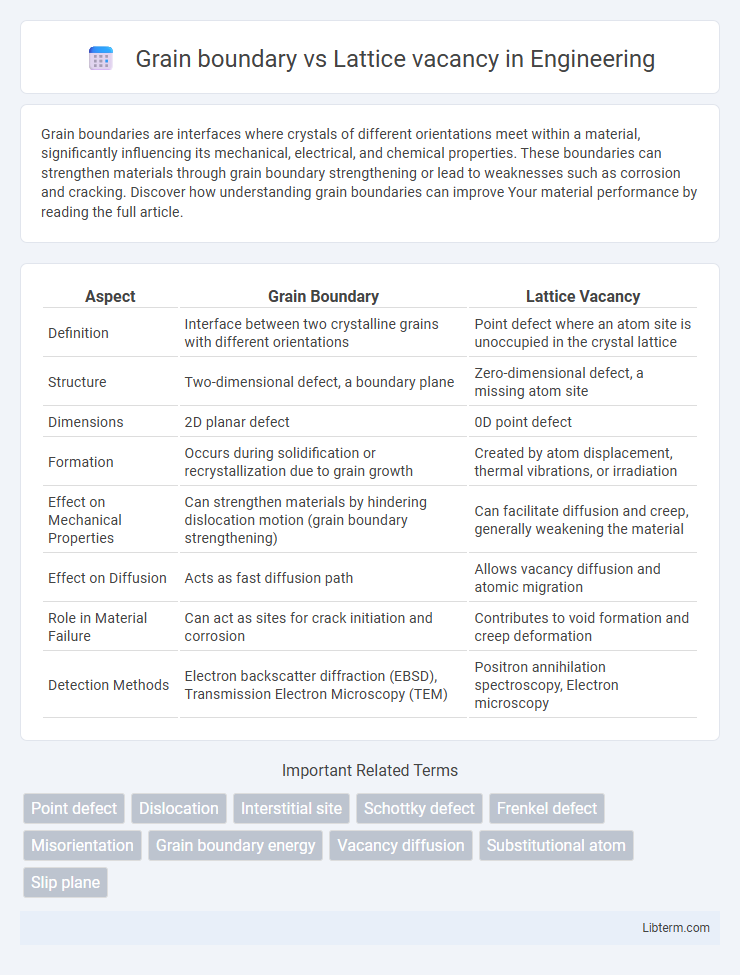
| Aspect | Grain Boundary | Lattice Vacancy |
|---|---|---|
| Definition | Interface between two crystalline grains with different orientations | Point defect where an atom site is unoccupied in the crystal lattice |
| Structure | Two-dimensional defect, a boundary plane | Zero-dimensional defect, a missing atom site |
| Dimensions | 2D planar defect | 0D point defect |
| Formation | Occurs during solidification or recrystallization due to grain growth | Created by atom displacement, thermal vibrations, or irradiation |
| Effect on Mechanical Properties | Can strengthen materials by hindering dislocation motion (grain boundary strengthening) | Can facilitate diffusion and creep, generally weakening the material |
| Effect on Diffusion | Acts as fast diffusion path | Allows vacancy diffusion and atomic migration |
| Role in Material Failure | Can act as sites for crack initiation and corrosion | Contributes to void formation and creep deformation |
| Detection Methods | Electron backscatter diffraction (EBSD), Transmission Electron Microscopy (TEM) | Positron annihilation spectroscopy, Electron microscopy |
Introduction to Crystal Defects
Grain boundaries are interfaces between differently oriented crystallites in polycrystalline materials, significantly impacting mechanical properties and diffusion behavior. Lattice vacancies are point defects characterized by absent atoms in the crystal lattice, influencing electrical conductivity and diffusion rates at the atomic level. Both defects alter the local atomic arrangement, playing critical roles in determining materials' strength, ductility, and diffusion mechanisms.
Defining Grain Boundaries
Grain boundaries are interfaces where crystals of different orientations meet within a polycrystalline material, significantly impacting mechanical and electrical properties. Unlike lattice vacancies, which are point defects involving missing atoms within the crystal lattice, grain boundaries represent two-dimensional defects that disrupt atomic arrangement over larger regions. These boundaries act as barriers to dislocation movement, affecting strength and diffusivity in metals and ceramics.
Understanding Lattice Vacancies
Lattice vacancies are point defects in a crystal structure where an atom site is unoccupied, significantly influencing material properties like diffusion and mechanical strength. These vacancies differ from grain boundaries, which are interfaces between differently oriented crystals, by affecting atomic mobility within grains rather than at grain interfaces. Understanding lattice vacancies enables control over phenomena such as creep, ionic conductivity, and sintering in metals and ceramics.
Origins of Grain Boundaries vs Lattice Vacancies
Grain boundaries originate from the misalignment and orientation differences between adjacent crystallites during the solidification or deformation of polycrystalline materials, forming interfaces where atomic arrangements are disrupted. Lattice vacancies arise from missing atoms in the crystal lattice, typically generated by thermal vibrations, irradiation, or plastic deformation, creating point defects within the atomic structure. While grain boundaries represent planar defects between grains, lattice vacancies are localized atomic-scale defects within a single crystal lattice.
Structural Differences Between the Two Defects
Grain boundaries are planar defects characterized by the interface where two differently oriented crystalline grains meet, causing a mismatch in lattice orientation across the boundary. Lattice vacancies are point defects involving missing atoms within a crystal lattice, resulting in localized disruptions without altering the overall lattice orientation. The structural difference lies in grain boundaries being two-dimensional interfacial regions affecting multiple atomic planes, whereas lattice vacancies are zero-dimensional defects confined to single atomic sites.
Impact on Material Properties
Grain boundaries act as barriers to dislocation motion, enhancing material strength through grain boundary strengthening while also serving as sites for impurity segregation, which can reduce corrosion resistance. Lattice vacancies facilitate atomic diffusion, influencing processes such as creep, sintering, and phase transformations that affect mechanical properties and material stability. The interaction between grain boundaries and lattice vacancies critically governs thermal and mechanical behavior, impacting ductility, hardness, and overall performance in metals and ceramics.
Role in Diffusion Mechanisms
Grain boundaries act as high-diffusivity pathways in polycrystalline materials, significantly enhancing atomic migration compared to lattice vacancies, which serve as point defects enabling atomic jumps within the crystal lattice. Diffusion along grain boundaries occurs faster due to the disrupted atomic structure and increased free volume, facilitating rapid solute or atom transport. In contrast, vacancies promote diffusion through vacancy-mediated atom exchanges, which are slower and depend on vacancy concentration and activation energy.
Influence on Mechanical Strength
Grain boundaries impede dislocation motion, significantly enhancing mechanical strength through grain boundary strengthening mechanisms like the Hall-Petch effect. Lattice vacancies, as point defects, can facilitate diffusion and dislocation climb, potentially reducing strength by promoting creep and plastic deformation. The interaction between grain boundaries and lattice vacancies critically influences the overall mechanical performance of polycrystalline materials.
Visualization and Detection Methods
Grain boundaries and lattice vacancies are critical defects within crystalline materials, distinguishable through advanced visualization and detection methods. Grain boundaries are typically visualized using Electron Backscatter Diffraction (EBSD) mapping and Transmission Electron Microscopy (TEM), which reveal orientation differences and boundary structures, while lattice vacancies require positron annihilation spectroscopy (PAS) and high-resolution TEM for detection at the atomic scale. Accurate identification of these defects enhances understanding of material properties such as strength and diffusion behavior.
Applications and Engineering Considerations
Grain boundaries significantly influence mechanical properties such as strength and corrosion resistance in polycrystalline materials, making them critical in applications like aerospace and automotive industries where durability is essential. Lattice vacancies play a vital role in diffusion processes, impacting semiconductor device fabrication and solid-state ionic conductors by affecting electrical and ionic conductivity. Engineering considerations include controlling grain size through heat treatment to optimize grain boundary density, while vacancy concentration is managed via doping and thermal annealing to tailor material performance.
Grain boundary Infographic
 libterm.com
libterm.com