Galvanization protects steel and iron by coating them with a layer of zinc, preventing rust and corrosion. This process extends the lifespan of metal products used in construction, automotive, and outdoor applications. Discover how galvanization can enhance the durability of your metal materials by reading the rest of the article.
Table of Comparison
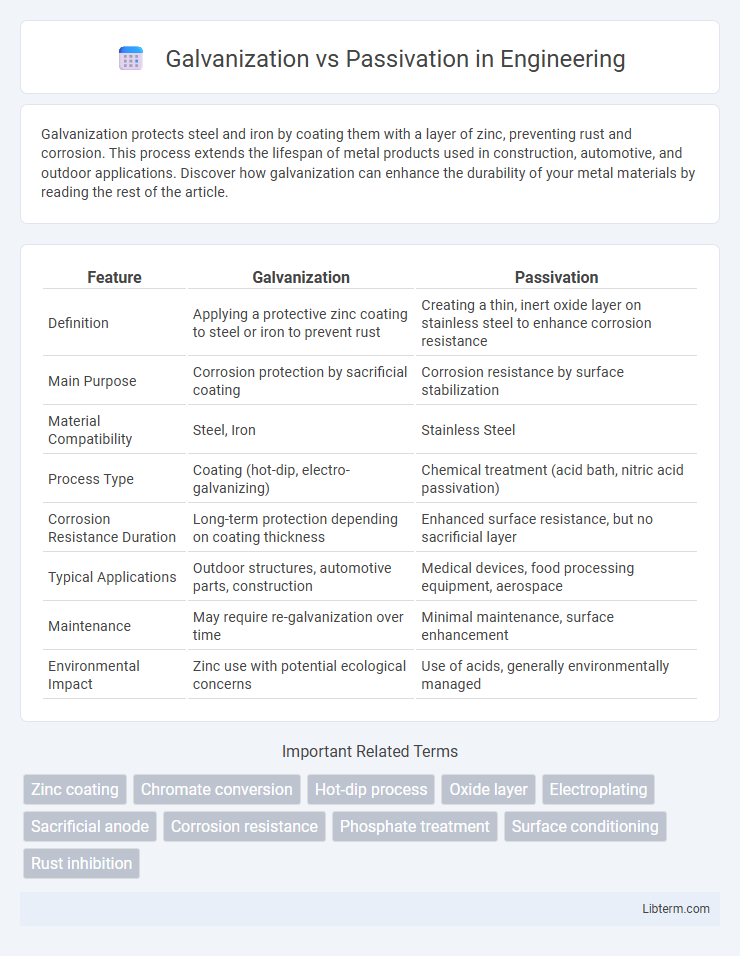
| Feature | Galvanization | Passivation |
|---|---|---|
| Definition | Applying a protective zinc coating to steel or iron to prevent rust | Creating a thin, inert oxide layer on stainless steel to enhance corrosion resistance |
| Main Purpose | Corrosion protection by sacrificial coating | Corrosion resistance by surface stabilization |
| Material Compatibility | Steel, Iron | Stainless Steel |
| Process Type | Coating (hot-dip, electro-galvanizing) | Chemical treatment (acid bath, nitric acid passivation) |
| Corrosion Resistance Duration | Long-term protection depending on coating thickness | Enhanced surface resistance, but no sacrificial layer |
| Typical Applications | Outdoor structures, automotive parts, construction | Medical devices, food processing equipment, aerospace |
| Maintenance | May require re-galvanization over time | Minimal maintenance, surface enhancement |
| Environmental Impact | Zinc use with potential ecological concerns | Use of acids, generally environmentally managed |
Introduction to Galvanization and Passivation
Galvanization involves coating steel or iron with a protective layer of zinc to prevent rust and corrosion through sacrificial protection. Passivation is a chemical treatment that enhances the natural oxide layer on metals like stainless steel, increasing corrosion resistance without adding a physical coating. Both processes improve metal durability but utilize distinct mechanisms--galvanization provides a physical barrier and sacrificial protection, whereas passivation strengthens the existing passive oxide layer.
What is Galvanization?
Galvanization is a metal protection process that involves coating steel or iron with a layer of zinc to prevent corrosion and rust. The zinc layer acts as a physical barrier and provides cathodic protection by corroding preferentially, thereby extending the lifespan of the underlying metal. This technique is widely used in construction, automotive, and manufacturing industries to enhance durability and resistance to environmental factors.
What is Passivation?
Passivation is a chemical process that enhances the corrosion resistance of metal surfaces by forming a thin, protective oxide layer. This oxide layer acts as a barrier, preventing oxygen and moisture from reaching the underlying metal, thus reducing oxidation and rust. Commonly applied to stainless steel, passivation typically involves treatment with nitric acid or citric acid to remove free iron and contaminants that can interrupt the protective film.
Chemical Processes Involved
Galvanization involves coating a metal, typically steel or iron, with a layer of zinc through electroplating or hot-dip methods, providing a sacrificial barrier that prevents corrosion by reacting with the environment before the base metal. Passivation chemically treats stainless steel or aluminum surfaces with acidic solutions, such as nitric or citric acid, to remove free iron and enhance the formation of a protective oxide layer, increasing corrosion resistance. Both processes rely on surface chemistry modifications but differ as galvanization adds a metallic coating while passivation alters the metal's surface oxide composition.
Materials Suitable for Each Process
Galvanization is most suitable for ferrous metals like steel and iron, providing robust corrosion resistance through a zinc coating. Passivation targets stainless steel and other non-ferrous metals, enhancing the naturally occurring oxide layer to prevent rust and degradation. Each process is selected based on the metal's composition and desired durability in corrosive environments.
Corrosion Resistance Comparison
Galvanization provides superior corrosion resistance by applying a protective zinc coating that sacrificially corrodes to protect the underlying metal, especially effective in harsh outdoor environments. Passivation enhances corrosion resistance primarily through the formation of a thin, inert oxide layer on stainless steel surfaces, reducing metal reactivity without adding a protective sacrificial layer. In environments with prolonged exposure to moisture and corrosive elements, galvanization generally offers longer-lasting protection, whereas passivation is ideal for maintaining stainless steel's corrosion resistance and appearance in less aggressive conditions.
Applications and Industries
Galvanization is widely utilized in automotive, construction, and infrastructure industries for corrosion resistance in steel components exposed to harsh environments, such as bridges and pipelines. Passivation finds applications primarily in electronics, aerospace, and medical device manufacturing, where stainless steel and other alloys require enhanced surface corrosion resistance without altering bulk properties. Both processes are critical in extending the lifespan and durability of metal products in sectors demanding high reliability and safety.
Cost and Economic Considerations
Galvanization generally incurs higher upfront costs due to the use of zinc coatings and specialized application processes, but it provides long-term corrosion protection that can reduce maintenance expenses and replacement frequency. Passivation, often applied to stainless steel, involves chemical treatments that are less expensive initially but may require more frequent inspections and repairs over time. Evaluating total lifecycle costs favors galvanization for environments prone to heavy corrosion, while passivation may be more cost-effective for less aggressive conditions.
Environmental Impact
Galvanization involves coating metal with a layer of zinc to prevent corrosion, which can lead to zinc runoff contaminating water sources and affecting aquatic ecosystems. Passivation enhances metal corrosion resistance by forming a protective oxide layer, reducing the need for harmful chemicals and minimizing environmental pollutants. Evaluating environmental impact, passivation generally offers a more eco-friendly approach due to lower toxic metal emissions and reduced waste generation compared to galvanization.
Choosing the Right Surface Treatment
Choosing the right surface treatment depends on the specific application and environmental conditions; galvanization provides a robust zinc coating that offers superior corrosion resistance for outdoor or industrial use, especially in harsh climates. Passivation, typically applied to stainless steel, enhances the natural oxide layer, improving corrosion resistance primarily in less aggressive environments and maintaining metal aesthetics. Evaluating factors such as exposure to moisture, chemical exposure, and mechanical wear ensures selection of the optimal treatment to maximize durability and performance.
Galvanization Infographic
 libterm.com
libterm.com