Phosphating is a chemical treatment process that enhances metal surfaces by creating a protective phosphate coating, improving corrosion resistance and paint adhesion. This technique is essential in industries such as automotive and manufacturing where durability and surface preparation are critical. Discover how phosphating can optimize your metal treatment processes by reading the full article.
Table of Comparison
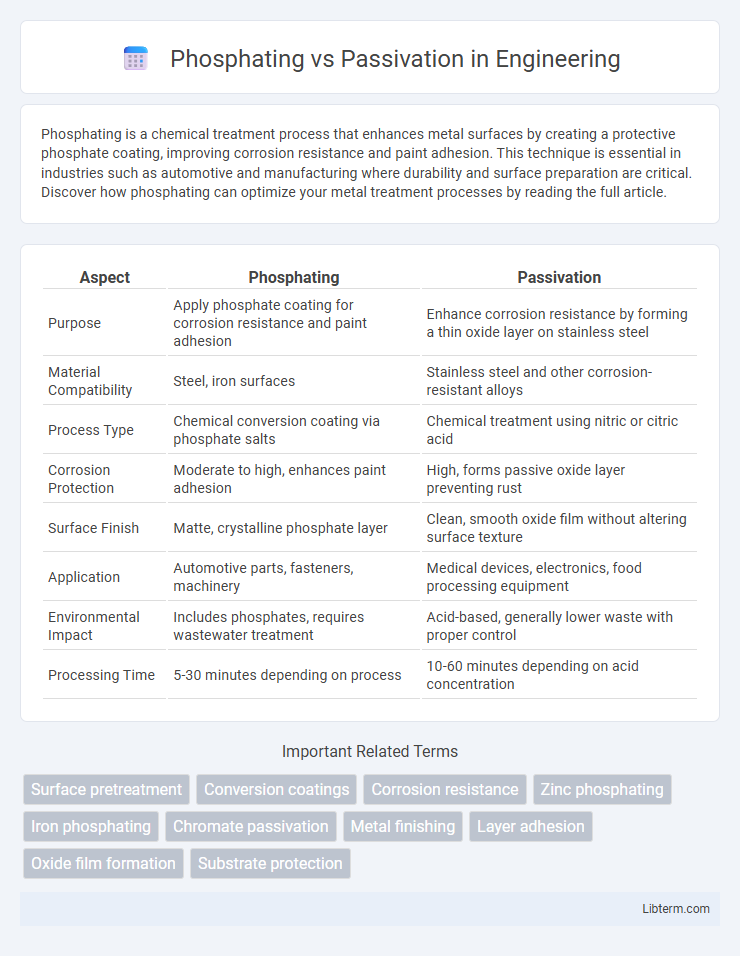
| Aspect | Phosphating | Passivation |
|---|---|---|
| Purpose | Apply phosphate coating for corrosion resistance and paint adhesion | Enhance corrosion resistance by forming a thin oxide layer on stainless steel |
| Material Compatibility | Steel, iron surfaces | Stainless steel and other corrosion-resistant alloys |
| Process Type | Chemical conversion coating via phosphate salts | Chemical treatment using nitric or citric acid |
| Corrosion Protection | Moderate to high, enhances paint adhesion | High, forms passive oxide layer preventing rust |
| Surface Finish | Matte, crystalline phosphate layer | Clean, smooth oxide film without altering surface texture |
| Application | Automotive parts, fasteners, machinery | Medical devices, electronics, food processing equipment |
| Environmental Impact | Includes phosphates, requires wastewater treatment | Acid-based, generally lower waste with proper control |
| Processing Time | 5-30 minutes depending on process | 10-60 minutes depending on acid concentration |
Introduction to Surface Treatments: Phosphating vs Passivation
Phosphating and passivation are essential surface treatments used to enhance corrosion resistance and improve adhesion for subsequent coatings on metal components. Phosphating involves applying a crystalline phosphate coating, primarily on steel and iron, to create a porous layer that promotes paint adherence and wear resistance. Passivation treats stainless steel by forming a thin, protective oxide layer that prevents oxidation and maintains the metal's corrosion-resistant properties without altering its surface texture.
Understanding the Basics: What is Phosphating?
Phosphating is a chemical process that applies a phosphate coating to metal surfaces, primarily steel, to enhance corrosion resistance, improve paint adhesion, and reduce friction. This conversion coating forms crystalline phosphate crystals that provide a protective barrier and promote better bonding for subsequent coatings or paints. Phosphating is widely used in automotive and manufacturing industries to prepare metal parts for further finishing processes.
Defining Passivation: Process and Purpose
Passivation is a chemical treatment process that enhances the corrosion resistance of metal surfaces by forming a thin, inert oxide layer, typically on stainless steel and other ferrous metals. This process involves immersing the metal in an acid solution, commonly nitric or citric acid, which removes free iron and contaminants, promoting the formation of a stable chromium-rich oxide film. The primary purpose of passivation is to protect metal parts from rust and environmental degradation, extending their durability and performance in industrial applications.
Key Differences Between Phosphating and Passivation
Phosphating involves applying a phosphate coating to metal surfaces to enhance corrosion resistance and improve paint adhesion, commonly used in steel treatment processes. Passivation is a chemical process that forms a thin oxide layer on metals like stainless steel, preventing oxidation and corrosion by creating a protective barrier. Key differences include phosphating adding a physical coating for better paint bonding, while passivation chemically alters the metal surface to naturally resist corrosion without adding material.
Chemical Processes Involved in Phosphating and Passivation
Phosphating involves the chemical conversion of a metal surface into a layer of insoluble crystalline phosphate salts through immersion in zinc, iron, or manganese phosphate baths, which enhances corrosion resistance and paint adhesion. Passivation uses chemical treatments, typically with nitric or citric acid, to remove free iron from stainless steel surfaces, forming a dense, inert oxide layer that protects against oxidation and rust. Both processes rely on controlled chemical reactions that modify the metal surface at a molecular level for improved durability and protective properties.
Applications and Industries Using Phosphating
Phosphating is widely used in automotive, construction, and appliance manufacturing industries due to its effective corrosion resistance and excellent paint adhesion properties. This surface treatment process is critical for enhancing the durability of steel and iron components in heavy machinery, automotive bodies, and household appliances. Unlike passivation, which is primarily applied to stainless steel for rust prevention, phosphating provides a robust phosphate coating that acts as a base for further coating or painting processes in metal fabrication.
Common Uses of Passivation in Industry
Passivation is widely used in the semiconductor, aerospace, and pharmaceutical industries to enhance corrosion resistance by forming a protective oxide layer on metal surfaces such as stainless steel and titanium. It is essential in medical device manufacturing to prevent contamination and prolong equipment lifespan. Unlike phosphating, which primarily provides a base for paint or lubrication, passivation focuses on improving metal durability and biocompatibility.
Advantages and Limitations: Phosphating vs Passivation
Phosphating offers superior corrosion resistance and enhanced paint adhesion by forming a crystalline phosphate layer, which is ideal for automotive and heavy machinery industries. Passivation provides a thin, inert oxide layer that improves stainless steel's corrosion resistance with minimal environmental impact but may be less effective on non-stainless alloys. Limitations of phosphating include hazardous waste generation and intricate process control, whereas passivation struggles with surface defects and requires precise chemical treatments for consistent results.
Choosing the Right Surface Treatment for Your Needs
Phosphating enhances corrosion resistance and paint adhesion by creating a crystalline phosphate coating, ideal for automotive and industrial metal parts requiring durability and primer bonding. Passivation removes surface contaminants and boosts corrosion resistance through forming a thin, inert oxide layer, commonly used for stainless steel and aluminum components in environments demanding high cleanliness and corrosion resistance. Selecting the right surface treatment depends on factors such as substrate material, environmental exposure, and functional requirements, with phosphating suited for heavy-duty coatings and passivation preferred for maintaining metal purity and corrosion resistance.
Summary: Phosphating vs Passivation Comparison
Phosphating creates a crystalline phosphate layer on metal surfaces, enhancing corrosion resistance and paint adhesion, while passivation forms a thin oxide film mainly to prevent rust on stainless steel. Phosphating is commonly used in automotive and industrial applications for improved coating durability, whereas passivation is essential for maintaining the stainless steel's corrosion-resistant properties. Both processes are surface treatments but differ in chemical composition, application purpose, and industries served.
Phosphating Infographic
 libterm.com
libterm.com