Work hardening enhances the strength and durability of metals by plastically deforming their crystalline structure, creating more dislocations that impede movement. This process is often used in manufacturing to improve material properties without altering chemical composition. Discover how understanding work hardening can optimize Your metalworking projects in the full article.
Table of Comparison
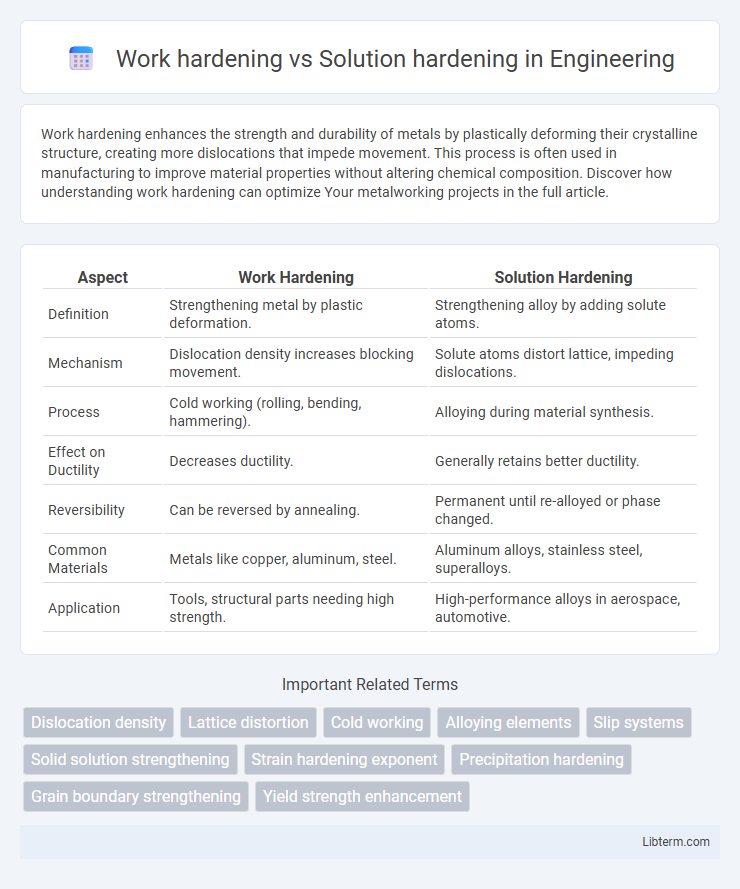
| Aspect | Work Hardening | Solution Hardening |
|---|---|---|
| Definition | Strengthening metal by plastic deformation. | Strengthening alloy by adding solute atoms. |
| Mechanism | Dislocation density increases blocking movement. | Solute atoms distort lattice, impeding dislocations. |
| Process | Cold working (rolling, bending, hammering). | Alloying during material synthesis. |
| Effect on Ductility | Decreases ductility. | Generally retains better ductility. |
| Reversibility | Can be reversed by annealing. | Permanent until re-alloyed or phase changed. |
| Common Materials | Metals like copper, aluminum, steel. | Aluminum alloys, stainless steel, superalloys. |
| Application | Tools, structural parts needing high strength. | High-performance alloys in aerospace, automotive. |
Introduction to Hardening Processes
Work hardening and solution hardening are key processes used to improve the mechanical properties of metals by increasing their strength and hardness. Work hardening, also known as strain hardening, enhances material strength through plastic deformation, causing dislocation movements that block further deformation. Solution hardening involves alloying metals with solute atoms that create lattice distortions, impeding dislocation motion and strengthening the material at the atomic level.
Defining Work Hardening
Work hardening, also known as strain hardening, is the process where a metal becomes stronger and harder due to plastic deformation, increasing dislocation density within its crystal structure. This mechanism differs from solution hardening, which relies on alloying elements dissolved in the base metal to impede dislocation motion. Understanding work hardening is crucial for improving metal strength through mechanical deformation rather than altering chemical composition.
Defining Solution Hardening
Solution hardening refers to the process of strengthening a metal by dissolving alloying elements into its crystal lattice, creating a solid solution that impedes dislocation movement and enhances mechanical properties. Unlike work hardening, which relies on plastic deformation to increase dislocation density, solution hardening improves hardness and strength through atomic-level substitution or interstitial placement. Common examples include adding carbon to iron to form steel or alloying aluminum with zinc, magnesium, or copper to achieve superior strength and corrosion resistance.
Key Mechanisms of Work Hardening
Work hardening, also known as strain hardening, occurs through the increased dislocation density within a metal's crystal structure as it undergoes plastic deformation, enhancing its strength and hardness. Solution hardening relies on the addition of alloying elements that create lattice distortions, impeding dislocation motion by atomic-scale stress fields. The key mechanisms of work hardening involve dislocation entanglement and interactions that create obstacles to further dislocation movement, thereby increasing the material's yield strength and tensile strength.
Key Mechanisms of Solution Hardening
Solution hardening increases material strength by dissolving alloying elements in the base metal, creating a solid solution that impedes dislocation motion. The key mechanisms include lattice distortions caused by atomic size differences and elastic strain fields from solute atoms, which hinder dislocation glide and improve hardness. This contrasts with work hardening, which relies on increasing dislocation density through plastic deformation.
Microstructural Changes During Hardening
Work hardening increases dislocation density within the metal's crystal structure, leading to strain-induced defects that impede further dislocation motion and enhance strength. Solution hardening involves alloying elements dissolving into the metal matrix, creating lattice distortions that obstruct dislocation movement at the atomic level. Microstructural changes during work hardening are primarily characterized by tangled dislocations and increased internal stress, while solution hardening results in a uniform solid solution with dispersed solute atoms causing localized strain fields.
Comparative Advantages of Work and Solution Hardening
Work hardening increases metal strength and hardness through plastic deformation, enhancing yield strength and tensile strength without altering chemical composition, making it ideal for shaping and bending processes. Solution hardening, on the other hand, improves material hardness by dissolving alloying elements into a solid solution, providing superior corrosion resistance and elevated temperature stability. Work hardening offers quick, cost-effective improvements in mechanical properties during fabrication, while solution hardening delivers enhanced long-term durability and reliability in harsh environments.
Industrial Applications of Each Hardening Method
Work hardening is widely used in industrial applications such as metal forming and machining to enhance strength and wear resistance through plastic deformation, improving durability in structural components and tools. Solution hardening, applied primarily in alloy production like stainless steel and aluminum alloys, increases hardness by dissolving alloying elements into the metal matrix, benefiting industries requiring corrosion resistance and high strength-to-weight ratios, such as aerospace and automotive sectors. Each method is selected based on operational requirements: work hardening excels in improving surface properties during fabrication, while solution hardening offers enhanced bulk mechanical properties after heat treatment.
Limitations and Challenges of Both Techniques
Work hardening can lead to increased brittleness and reduced ductility, limiting its effectiveness in applications requiring significant deformation after strengthening. Solution hardening faces challenges such as the limited solubility of alloying elements in the base metal and potential phase instability at high temperatures. Both techniques struggle with balancing strength enhancement while maintaining adequate toughness and workability in engineering components.
Choosing the Optimal Hardening Method
Choosing the optimal hardening method depends on the specific material properties and application requirements. Work hardening enhances strength through plastic deformation, ideal for metals like copper and aluminum that benefit from increased dislocation density without altering composition. Solution hardening involves alloying by dissolving elements into a metal matrix, providing superior strength and hardness for materials like stainless steel and aluminum alloys under high-temperature conditions.
Work hardening Infographic
 libterm.com
libterm.com