The equilibrium state represents a condition where opposing forces or influences are balanced, resulting in stability within a system. Understanding this concept is crucial in fields ranging from physics and chemistry to economics and ecology, as it explains how systems maintain consistency despite external changes. Explore the rest of this article to deepen your insight into how equilibrium shapes various natural and engineered processes.
Table of Comparison
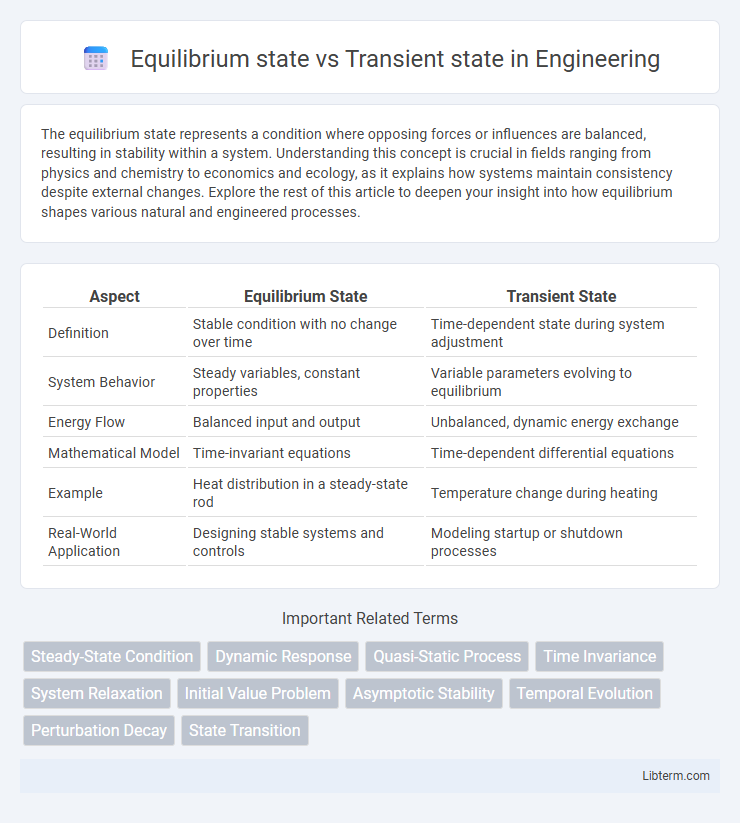
| Aspect | Equilibrium State | Transient State |
|---|---|---|
| Definition | Stable condition with no change over time | Time-dependent state during system adjustment |
| System Behavior | Steady variables, constant properties | Variable parameters evolving to equilibrium |
| Energy Flow | Balanced input and output | Unbalanced, dynamic energy exchange |
| Mathematical Model | Time-invariant equations | Time-dependent differential equations |
| Example | Heat distribution in a steady-state rod | Temperature change during heating |
| Real-World Application | Designing stable systems and controls | Modeling startup or shutdown processes |
Introduction to Equilibrium and Transient States
Equilibrium state refers to a condition where all system variables remain constant over time, indicating no net change in energy, mass, or momentum within the system. Transient state describes the period during which these variables change as the system moves from an initial condition toward equilibrium. Understanding these states is crucial in fields like thermodynamics, fluid mechanics, and chemical engineering to analyze system stability and response times.
Defining Equilibrium State
Equilibrium state refers to a condition where all variables in a system remain constant over time, indicating no net change in energy, mass, or momentum within the system. This state occurs when forward and reverse processes balance perfectly, resulting in steady-state conditions. In contrast, transient state involves temporary changes as the system evolves toward equilibrium, characterized by variable fluctuations before stabilization.
Understanding Transient State
Transient state refers to the period during which a system undergoes change before reaching equilibrium, characterized by time-dependent variations in variables such as temperature, pressure, or concentration. Understanding transient state involves analyzing dynamic responses and rates of change, crucial for designing control systems and predicting system behavior under non-steady conditions. Accurately modeling transient state ensures effective management of processes in engineering, chemical reactions, and thermodynamics.
Key Differences Between Equilibrium and Transient States
Equilibrium state refers to a condition where all variables in a system remain constant over time, indicating that no net change occurs in properties such as temperature, pressure, or concentration. Transient state describes the period during which these variables experience change as the system moves toward equilibrium. The key differences lie in the stability of system parameters: equilibrium represents a steady state with no temporal variation, while transient state involves dynamic shifts and system responses to external or internal changes.
Characteristics of Equilibrium State
The equilibrium state is characterized by stable conditions where system variables remain constant over time, with no net change in energy, mass, or momentum. In this state, all driving forces are balanced, and the system experiences no spontaneous fluctuations or gradients. Equilibrium conditions facilitate predictable and steady behavior, making it critical for analyzing thermodynamic and physical systems.
Characteristics of Transient State
The transient state is characterized by its dynamic nature where system variables such as temperature, pressure, or concentration change with time until reaching equilibrium. This period exhibits non-steady behavior, often accompanied by fluctuating rates of heat transfer, mass flow, or chemical reactions. Understanding transient state dynamics is essential for designing processes that require controlled startup, shutdown, or response to disturbances.
Examples of Equilibrium and Transient States in Real Systems
Equilibrium states occur when a system's properties remain constant over time, such as a chemical reaction at equilibrium where reactant and product concentrations are stable. Transient states appear during processes like the warming of a room after the heater is switched on, where temperature continuously changes before reaching equilibrium. Examples include thermal equilibrium in a cup of coffee left on a table versus the transient cooling phase shortly after it is poured.
Importance in Engineering and Science
Equilibrium state represents a condition where a system's variables remain constant over time, enabling engineers and scientists to simplify complex analyses and design stable systems. Transient state captures the dynamic response during a system's change from one equilibrium to another, providing critical insights for controlling processes and predicting performance under variable conditions. Understanding both states is vital for accurate modeling, optimization, and ensuring safety in disciplines such as thermodynamics, fluid mechanics, and electrical engineering.
Factors Influencing State Transition
Factors influencing the transition from equilibrium state to transient state include external disturbances, system parameter changes, and initial conditions. Variations in temperature, pressure, or concentration gradients drive the system away from equilibrium, triggering transient dynamics. System inertia and feedback mechanisms also affect the rate and stability of the state transition.
Summary and Practical Implications
Equilibrium state in a system occurs when all variables remain constant over time, indicating a balance between competing processes, while transient state represents the period of change as the system moves toward equilibrium. Understanding the transient state is crucial for designing processes that require time-dependent control, such as chemical reactors or thermal systems, where reaching steady conditions ensures optimal performance and safety. Practical implications include predicting system behavior during startup or shutdown phases and improving control strategies to minimize unwanted fluctuations and achieve stable operation efficiently.
Equilibrium state Infographic
 libterm.com
libterm.com